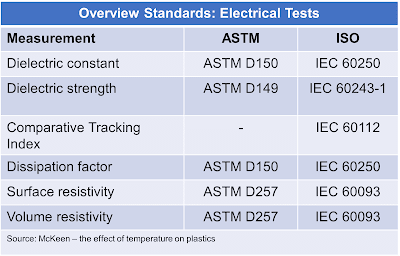
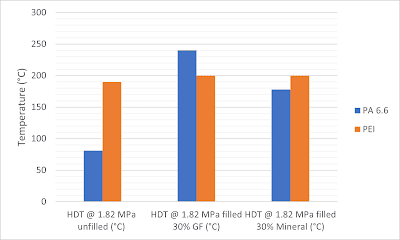
|
| Ocean Plastics - Episode 2 |
Hello and welcome back to a new post. Today we continue with Episode 2 of “Ocean Plastics”.
Here is the link to Episode1.
Media and NGOs are pushing the topic of ocean plastic contamination in their publications up and down in turn for attention and more funding. It was already proven by Mr. DeArmitt and others that the science was ignored in most cases and even pictures of turtles were photo shopped to make it even more dramatic.
Due to the low density of plastics (0.9 -1 g/ccm) they are floating on and right beneath the water surface which allows them to be relatively easy to bee seen.
Since plastics represent only 1% of the material and waste, following key question opens up to me:
What else is in and on the ground of our seas?
Turns out a lot! Let us examine in detail.
Oil
Oil represents a large share of sea pollutants. It was estimated that there are 6,300 wrecks, sunk in World War II, rusting at sea for more than 70 years. Researchers estimate the amount of oil left in them at up to 15 million tons. If the oil storage of the wrecks starts to burst, oil spills will destroy all the plant and animal life of a particular region. Additionally to the 6,300 potentially polluting wrecks around the world, there are 1,583 tank vessels which are a ticking bomb too. Apart from oil, a warship itself contains huge quantities of bronze, brass, copper, and other non-ferrous metals. Interesting is the low-background steel from wrecks sunk before 1945 since this type of steel is not emitting ionizing radiation.
Heavy metals
Metals with a density greater than 3.5 g/ccm can be classed as heavy metals. In this category fall copper, nickel, cadmium, iron, lead, mercury and zinc. Out of the aforementioned metals, lead, mercury and cadmium are the most concerning for sea life. Due to increased industrial activity, heavy metals get into the atmosphere and from there they end up in the oceans.
Radioactive waste
Mr. Calmet investigated back in 1989 the radioactive waste disposal in oceans and found out that thirteen countries used ocean disposal to get rid of their radioactive waste. 200,000 tons in nuclear waste was approximated which derives mainly from the medical, research and nuclear industry.
Airplanes
Particularly in the WWII area, hundreds of airplanes found their last station on the bottom of the sea. Similar to ship wrecks, they have oil and ammunition which can impact sea life.
Ocean dumping in the United States prior to 1972
The US National Academy of Sciences estimated in 1968 the following annual volumes of ocean dumping by vessel or pipes:
-100 million tons of petroleum products;
-two to four million tons of acid chemical wastes from pulp mills;
-more than one million tons of heavy metals in industrial wastes; and
-more than 100,000 tons of organic chemical wastes.
Conclusion
The "out of sight, out of mind” attitude for dumping waste into our ocean is wrong. Also, blaming plastics to be the number one littering source for our oceans is wrong too. The data speaks a clear language. There is more and more ideological thinking involved in such anti-plastics topics and too less decision making based on facts. Plastics are part of our solution and are not the problem.
Thanks for reading and #findoutaboutplastics
Greetings,
Herwig
Interested to talk with me about your plastic selection, sustainability, and part design needs - here you can contact me
Literature:
[1] https://www.linkedin.com/pulse/plastic-fact-over-fiction-chris-dearmitt-phd-frsc-fimmm/
[2] https://www.newscientist.com/article/mg20727761-600-why-wartime-wrecks-are-slicking-time-bombs/
[3] https://www.youtube.com/watch?v=XgdE55ZAFvs&t=4s
[4] Michael F. Ashby: Materials and the Environment: Eco-informed Material Choice
[5] https://assembly.coe.int/nw/xml/XRef/Xref-XML2HTML-en.asp?fileid=18077&lang=en
[6] https://www.envirotech-online.com/news/water-wastewater/9/breaking-news/why-is-there-heavy-metal-in-our-oceans/32291
[7]https://www.todayifoundout.com/index.php/2020/12/the-bizarre-market-for-old-battleship-steel/
[8] https://inis.iaea.org/search/searchsinglerecord.aspx?recordsFor=SingleRecord&RN=21044010
[9] https://www.epa.gov/ocean-dumping/learn-about-ocean-dumping#Before
[10] https://www.findoutaboutplastics.com/2018/08/what-media-does-not-tell-you-about.html
[11] https://plasticsparadox.com/